CAM and National Health Care Systems
strong global interest in CAM is demonstrated by transnational surveys supported or directly organized by governmental and non-governmental organizations, WHO (177), the Institute of Medicine (USA), as well as, for example, the European Commission (see page 97).
Individual governments are gradually reacting to this trend by trying to legally define non-medical treatments and to develop the rules for their provision. The progress in different countries varies considerably in this respect (178).
In Asian countries, such as China or South Korea, where natural healing practices have a long tradition, TM/CAM is fully integrated into public healthcare: TM/CAM is included in the national drug policy; providers and manufacturers are registered; TM/CAM therapies are provided in hospitals; TM/CAM treatment is covered by patients’ health insurance; research is publicly funded; and there are TM/CAM educational programs.
Some developed countries, such as Canada, the USA and Australia, have developed their own models for legalizing and regulating the most used CAM therapies. The models are either run by the government (e.g. a specialized department within the Ministry of Health) or non-governmental organizations (e.g. national professional associations of practitioners). The CAM therapies can be provided by either doctors or non-medical doctors that meet eligibility criteria and are properly registered. There are specialized research centres dedicated to various CAM methods that also provide the public with information on the efficacy and safety of individual methods. One example is The National Center for Complementary and Integrative Health – NCCIH in the USA. These countries also have working education and certification systems available for CAM providers; for some CAM areas, there are even university degree programmes and titles. Familiarization with selected CAM therapies is included in the study plans of a significant number of medical faculties.
In most developed European countries, the integration of CAM into public healthcare has not advanced as much. However, there are already national and European professional associations, in particular for CAM practitioners (EUROCAM, EFCAM, CAMDOC, etc.), specialized CAM departments at major European universities, and an advance in multi-disciplinary research. This means that there is a growing amount of reliable information on the effects of CAM therapies. This can be used as a base for considering different models for legalizing and integrating CAM therapies into public healthcare. In recent years, the EU has seen a growing number of patients, doctors and non-medical CAM providers calling for a coordinated and strategic pan-European approach to CAM, in order to ensure affordable CAM care of adequate quality for all European citizens.
CAM is also reimbursed by certain health insurance companies. There is an overview of, mainly European, health insurance companies that cover CAM (including energy therapies) in the chapter Recommended Links on page 222.
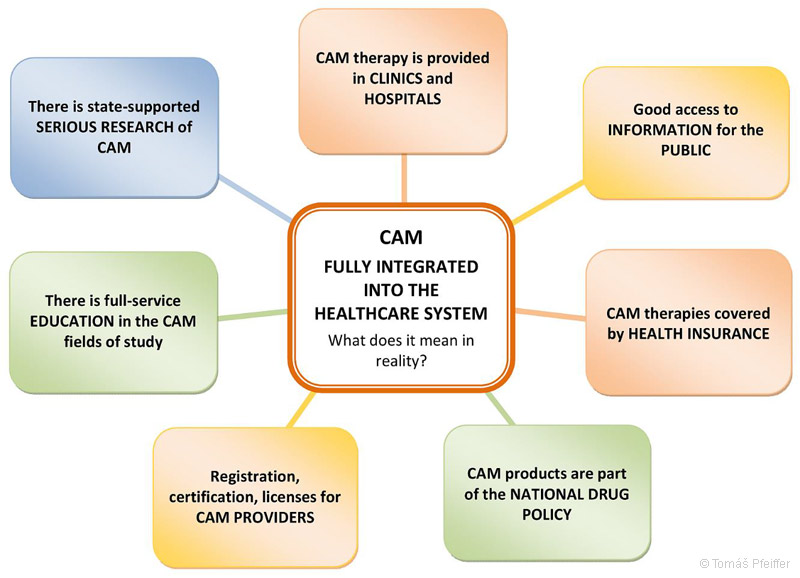
Figure 22 – What does it mean when complementary and alternative medicine (CAM) is fully integrated into the health care system?
The picture shows a situation that is currently, for example, in China or South Korea.
Countries such as Switzerland, USA, Canada, Australia, the UK or Germany are in differently advanced phases of the gradual integration of CAM into the system. In these countries, some of the above mentioned points are fulfilled completely, some partially.
Author of the schema – KoS.
Source: WHO. WHO Traditional Medicine Strategy 2002–2005 [online]. http://www.wpro.who.int/health_technology/book_who_traditional_medicine_strategy_2002_2005.pdf



