CAM Research Results
CAM Research Results in the Context of Medical Efficacy Assessment
Modern medicine has been developing since the mid-19th century when doctors began to address the question of the evidence of their empirical experience. There has been a growing need to demonstrate the efficacy of diagnostic and therapeutic procedures. With the development of physics, chemistry, biology, and the technical expansion of civilization, the current form of medicine is gradually developing hand in hand with the development of science and its increasing focus on technology. The evidence requirement of a given diagnostic or therapeutic procedure has become a focal point in medicine creating what we know as evidence-based medicine (EBM). The term EBM originated in the United States as a response to appeals from insurance companies, when there was a need for a monetary expression of the particular medical act.
Health insurance in the Czech Republic is based on the so-called solidarity principle (based on the German Bismarck health insurance system); each citizen has a legal obligation to contribute a certain amount of money to their health insurance. The money earmarked for health insurance goes to the state budget and the state, respectively the Ministry of Health, co-operates with providers of health insurance to decide how the money will be redistributed throughout the system. Each year, approximately CZK 300 billion flows through the system.
Educational literature from CTU (Czech Technical University) defines evidence-based medicine (EBM) as: "Conscious, transparent and judicious use of the best available evidence in decision-making regarding the care of each individual patient. The recommendation to exclusively use scientifically validated procedures was roughly formulated in the 1970s, but it was not until the 1990s that it became more widespread. (19)
EBM according to Jiří Heřt: "This new form of medicine was created in the US, primarily due to pressure from insurance companies. The principles of EBM were mainly elaborated by D. L. Sackett. According to his definition, EBM is a "care that integrates clinical experience with the patients‘ interests and scientific evidence on the efficacy of a treatment". It is clear from his definition, and from the way it is promoted and applied today, that EBM is not merely about blindly adhering to proven efficacy results, but also about respecting the clinical and personal experience of the doctor, as well as the patient and his or her opinion and interests.“20 (20)
Evidence-based medicine, or EBM, is based on the concept of evidence hierarchy, where the highest level within the evidence hierarchy is meta-analysis, and the lowest level is an individual observation / case study.21 (see Figure 11)
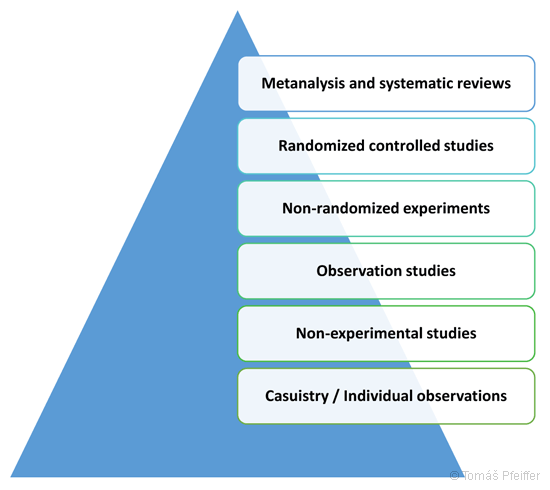
Figure 11 – Hierarchy of evidence for evidence-based medicine (EBM). The highest level within the evidence hierarchy is meta-analysis and the lowest level is individual observations / case reports.
Meta-analysis and systematic reviews – literally "an analysis of analyses" is a statistical summary of the results of previously published studies. The term was coined by the German sociologist Glass in 1976 and was soon adopted by various other disciplines, particularly medicine and clinical research. Glass considered it crucial to integrate the results of large numbers of analyses, i.e. to make the large quantities of results from various studies and analyses accessible within a single study, which experts could subsequently use as a reliable advisor. For example, meta-analysis can help strengthen the evidence of efficacy when individual studies are too small or have other limitations. A meta-analysis may also give rise to new areas to be considered in future clinical trials. A team of statisticians and representatives of medicine is required to conduct a meta-analysis. In practice meta-analyses often compares two types of treatment. (22) Here is a specific example of a meta-analysis that compares CAM and conventional medicine:
In 2004, 145 randomized controlled CAM studies in the database Cochrane (a non-profit NGO that performs independent medical tests, studies and meta-analyses) were analyzed. The results were then compared with a similar analysis of 1,016 general medical reviews. The data for this analysis was also from 2004. One example of the results of this analysis is that the efficacy of CAM is only a few percent lower than that of general medicine, and that it has a positive or potentially positive effect in 37.2% of the cases.
| (Possible) positive effect | Likely to have no effect | Likely to be harmful | Insufficient evidence | |
| CAM | 37,2% | 4,8% | 0,69% | 56,6% |
| Medicine in general | 44,4% | 0,98% | 7% | 47,8% |
Chart 2 – Results from the database Cochrane
Source: EUROCAM. CAM 2020 – The contribution of Complementary and Alternative Medicine to sustainable healthcare in Europe [online]. ehtpa.eu/pdf/CAM2020-FINAL.pdf.
Educational material from CTU defines the Cochrane Collaboration as an independent non-profit organization that aims to provide accessible information on the outcomes (effects) of health interventions. It is mainly active in preparing, preserving and disseminating systematic studies of randomized controlled trials (RCTs) in the field of health care. (23)
In his publication from 2010, Jiří Heřt says about the database Cochrane: ‘If we want to manage the results of clinical trials, we need to rely on the organization which is most respected for its abilities in assessing the quality and the results of medical studies in the field of both scientific and alternative medicine. This organization, the "Cochrane Collaboration", was founded in 1993 by Archie Cochrane, a former war surgeon, later a professor of lung disease. Today, the Cochrane Collaboration includes twelve scientific centres around the world and 10,000 scientists from 90 countries work voluntarily within the organization. These experts analyze published studies, and provide systematic overviews on the treatment outcomes of various diseases. This book often refers to conclusions reached by the Cochrane Collaboration.’ (24)
Heřt further states in his publication that: “Most pseudoscientific experiments are not carried out by experienced scientists at serious workplaces, and their work is not published in recognized, peer-reviewed journals.” (25)
Randomized controlled studies – study participants that are randomly divided into an intervention group (which is administered the test drug) and a control group (which is administered a placebo or a different medication).
Double-blind controlled studies – neither the patients nor the doctors assessing the treatment (double-blind studies) are familiar with group assignments; this kind of study is considered to be the golden scientific standard.
Non-randomized experiments – an observation of a given sample (e.g. population) without randomization that is performed to confirm / rebut a hypothesis; the conditions under which the experiment is performed are manipulated during the experiment; it should be possible to repeat the experiment at any time.
Observation study – a type of epidemiological study based on observations of the natural development of a given phenomenon in a particular population or a sample of a population.
Non-experimental studies – include: case studies, correlation research, comparative research and longitudinal research:
- case studies – research on a particular group or individual, it is generally not possible to extrapolate the results of a case studies to an entire population
- correlation research – compares two variables to see if there is a correlation between them (e.g. students‘ school results and the amount of attention received from the teachers)
- comparative research – compares specific subsets based on selected characteristics
- longitudinal research – research performed on a selected sample of the population over a longer period of time, usually several years (26)
Individual observations / case reports – the experiences of individual doctors / patients; a case report is a medical record mapping a diagnosis
Summary: The development of modern medicine began in the early 19th century; the concept of EBM, evidence-based medicine, was created in the 1970s following an appeal from insurers – as way to quantify individual healthcare procedures financially. There is a certain hierarchy of evidence within EBM – meta-analyses rank highest and the weakest form of evidence is case reports or individual observations. However, according to Heřt, the patient‘s personality and the doctor‘s opinion should also be taken into account when evaluating a therapeutic treatment. The Cochrane Collaboration is one of the most relevant and respected scientific sources and is governed by the principles of EBM. One of the studies provided by the Cochrane database compares the efficacy of medicine and CAM – the table with the results is one of the strongest arguments in favour of CAM (see Table 2).
If the therapeutic method / approach can not be evaluated according to EBM parameters, should it be considered non-existent?



